- Isotopes Of Elements Have Different
- Periodic Table Of Elements Isotopes
- Isotope Element Chart
- Isotopes Of Elements Diagram
- Isotopes Of Elements Have Different Brainly
Isotopes (Stable) for all the elements in the Periodic Table Isotopes (Stable) of the elements Up to date, curated data provided by Mathematica 's ElementData function from Wolfram Research, Inc.

Isotope Relative Atomic Mass Isotopic Composition Standard Atomic Weight Notes: 1: H: 1: 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m: D: 2: 2.014 101 778 12(12) 0.000 115(70) T: 3. This chemistry video tutorial answers the question - what are isotopes? Isotopes are substances that are composed of the same element but consist of differe. Theoretically, all five can decay into isotopes of element 72 (hafnium) by alpha emission, but only 180 W has been observed to do so. The other naturally occurring isotopes have not been observed to decay, and lower bounds for their half lives have been established: 182 W, t 1/2 7.7×10 21 years 183 W, t 1/2 4.1×10 21 years 184 W, t 1/2. 1 H, 2 H, 3 H, 4 H, 5 H, 6 H, 7 H. 2 He, 3 He, 4 He, 5 He, 6 He, 7 He, 8.

The periodic table is an arrangment of the chemical elements ordered by atomic number so that periodic properties of the elements (chemical periodicity) are made clear.
Isotopes Of Elements Have Different

Explore the isotope and NMR properties of the chemical elements through this periodic table
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period 1 | Hydrogen | Helium | |||||||||||||||||
| 2 | Lithium | Beryllium | Boron | Carbon | Nitrogen | Oxygen | Fluorine | Neon | |||||||||||
| 3 | Sodium | Magnesium | Aluminium | Silicon | Phosphorus | Sulfur | Chlorine | Argon | |||||||||||
| 4 | Potassium | Calcium | Scandium | Titanium | Vanadium | Chromium | Manganese | Iron | Cobalt | Nickel | Copper | Zinc | Gallium | Germanium | Arsenic | Selenium | Bromine | Krypton | |
| 5 | Rubidium | Strontium | Yttrium | Zirconium | Niobium | Molybdenum | Tc☢ Technetium | Ruthenium | Rhodium | Palladium | Silver | Cadmium | Indium | Tin | Antimony | Tellurium | Iodine | Xenon | |
| 6 | Caesium | Barium | * | Lutetium | Hafnium | Tantalum | Tungsten | Rhenium | Osmium | Iridium | Platinum | Gold | Mercury | Thallium | Lead | Bismuth | Po☢ Polonium | At☢ Astatine | Rn☢ Radon |
| 7 | Fr☢ Francium | Ra☢ Radium | ** | Lr☢ Lawrencium | Rf☢ Rutherfordium | Db☢ Dubnium | Sg☢ Seaborgium | Bh☢ Bohrium | Hs☢ Hassium | Mt☢ Meitnerium | Ds☢ Darmstadtium | Rg☢ Roentgenium | Cn☢ Copernicium | Nh☢ Nihonium | Fl☢ Flerovium | Mc☢ Moscovium | Lv☢ Livermorium | Ts☢ Tennessine | Og☢ Oganesson |
| *Lanthanoids | * | Lanthanum | Cerium | Praseodymium | Neodymium | Pm☢ Promethium | Samarium | Europium | Gadolinium | Terbium | Dysprosium | Holmium | Erbium | Thulium | Ytterbium | ||||
| **Actinoids | ** | Ac☢ Actinium | Th☢ Thorium | Pa☢ Protactinium | U☢ Uranium | Np☢ Neptunium | Pu☢ Plutonium | Am☢ Americium | Cm☢ Curium | Bk☢ Berkelium | Cf☢ Californium | Es☢ Einsteinium | Fm☢ Fermium | Md☢ Mendelevium | No☢ Nobelium | ||||
The standard form of the periodic table shown here includes periods (shown horizontally) and groups (shown vertically). The properties of elements in groups are similar in some respects to each other.
There is no one single or best structure for the periodic table but by whatever consensus there is, the form used here is very useful and the most common. The periodic table is a masterpiece of organised chemical information and the evolution of chemistry's periodic table into the current form is an astonishing achievement.
Since 1931, the Commission regularly publishes the critical evaluation of isotopic abundances of elements. These values form basis for the standard atomic weights of elements. Below is the current table of Isotopic Compositions of the Elements.
| Z | E | Element | A | Representative isotopic composition | Notes |
|---|---|---|---|---|---|
| 1 | H | hydrogen | 1 | [0.999 72, 0.999 99] | m |
| 2 | [0.000 01, 0.000 28] | ||||
| 2 | He | helium | 3 | 0.000 002(2) | |
| 4 | 0.999 998(2) | ||||
| 3 | Li | lithium | 6 | [0.019, 0.078] | m |
| 7 | [0.922, 0.981] | ||||
| 4 | Be | beryllium | 9 | 1 | |
| 5 | B | boron | 10 | [0.189, 0.204] | m |
| 11 | [0.796, 0.811] | ||||
| 6 | C | carbon | 12 | [0.9884, 0.9904] | |
| 13 | [0.0096, 0.0116] | ||||
| 7 | N | nitrogen | 14 | [0.995 78, 0.996 63] | |
| 15 | [0.003 37, 0.004 22] | ||||
| 8 | O | oxygen | 16 | [0.997 38, 0.997 76] | |
| 17 | [0.000 367, 0.000 400] | ||||
| 18 | [0.001 87, 0.002 22] | ||||
| 9 | F | fluorine | 19 | 1 | |
| 10 | Ne | neon | 20 | 0.9048(3) | |
| 21 | 0.0027(1) | ||||
| 22 | 0.0925(3) | ||||
| 11 | Na | sodium | 23 | 1 | |
| 12 | Mg | magnesium | 24 | [0.7888, 0.7905] | |
| 25 | [0.099 88, 0.100 34] | ||||
| 26 | [0.1096, 0.1109] | ||||
| 13 | Al | aluminium | 27 | 1 | |
| 14 | Si | silicon | 28 | [0.921 91, 0.923 18] | |
| 29 | [0.046 45, 0.046 99] | ||||
| 30 | [0.030 37, 0.031 10] | ||||
| 15 | P | phosphorus | 31 | 1 | |
| 16 | S | sulfur | 32 | [0.9441, 0.9529] | |
| 33 | [0.007 29, 0.007 97] | ||||
| 34 | [0.0396, 0.0477] | ||||
| 36 | [0.000 129, 0.000 187] | ||||
| 17 | Cl | chlorine | 35 | [0.755, 0.761] | m |
| 37 | [0.239, 0.245] | ||||
| 18 | Ar | argon | 36 | [0.0000, 0.0207] | |
| 38 | [0.000, 0.043] | ||||
| 40 | [0.936, 1.000] | ||||
| 19 | K | potassium | 39 | 0.932 581(44) | |
| 40 | 0.000 117(1) | ||||
| 41 | 0.067 302(44) | ||||
| 20 | Ca | calcium | 40 | 0.969 41(156) | g |
| 42 | 0.006 47(23) | ||||
| 43 | 0.001 35(10) | ||||
| 44 | 0.020 86(110) | ||||
| 46 | 0.000 04(3) | ||||
| 48 | 0.001 87(21) | ||||
| 21 | Sc | scandium | 45 | 1 | |
| 22 | Ti | titanium | 46 | 0.0825(3) | |
| 47 | 0.0744(2) | ||||
| 48 | 0.7372(3) | ||||
| 49 | 0.0541(2) | ||||
| 50 | 0.0518(2) | ||||
| 23 | V | vanadium | 50 | 0.002 50(10) | |
| 51 | 0.997 50(10) | ||||
| 24 | Cr | chromium | 50 | 0.043 45(13) | |
| 52 | 0.837 89(18) | ||||
| 53 | 0.095 01(17) | ||||
| 54 | 0.023 65(7) | ||||
| 25 | Mn | manganese | 55 | 1 | |
| 26 | Fe | iron | 54 | 0.058 45(105) | |
| 56 | 0.917 54(106) | ||||
| 57 | 0.021 19(29) | ||||
| 58 | 0.002 82(12) | ||||
| 27 | Co | cobalt | 59 | 1 | |
| 28 | Ni | nickel | 58 | 0.680 769(190) | r |
| 60 | 0.262 231(150) | ||||
| 61 | 0.011 399(13) | ||||
| 62 | 0.036 345(40) | ||||
| 64 | 0.009 256(19) | ||||
| 29 | Cu | copper | 63 | 0.6915(15) | r |
| 65 | 0.3085(15) | ||||
| 30 | Zn | zinc | 64 | 0.4917(75) | r |
| 66 | 0.2773(98) | ||||
| 67 | 0.0404(16) | ||||
| 68 | 0.1845(63) | ||||
| 70 | 0.0061(10) | ||||
| 31 | Ga | gallium | 69 | 0.601 08(50) | |
| 71 | 0.398 92(50) | ||||
| 32 | Ge | germanium | 70 | 0.2052(19) | |
| 72 | 0.2745(15) | ||||
| 73 | 0.0776(8) | ||||
| 74 | 0.3652(12) | ||||
| 76 | 0.0775(12) | ||||
| 33 | As | arsenic | 75 | 1 | |
| 34 | Se | selenium | 74 | 0.0086(3) | r |
| 76 | 0.0923(7) | ||||
| 77 | 0.0760(7) | ||||
| 78 | 0.2369(22) | ||||
| 80 | 0.4980(36) | ||||
| 82 | 0.0882(15) | ||||
| 35 | Br | bromine | 79 | [0.505, 0.508] | |
| 81 | [0.492, 0.495] | ||||
| 36 | Kr | krypton | 78 | 0.003 55(3) | |
| 80 | 0.022 86(10) | ||||
| 82 | 0.115 93(31) | ||||
| 83 | 0.115 00(19) | ||||
| 84 | 0.569 87(15) | ||||
| 86 | 0.172 79(41) | ||||
| 37 | Rb | rubidium | 85 | 0.7217(2) | g |
| 87 | 0.2783(2) | ||||
| 38 | Sr | strontium | 84 | 0.0056(2) | |
| 86 | 0.0986(20) | ||||
| 87 | 0.0700(20) | ||||
| 88 | 0.8258(35) | ||||
| 39 | Y | yttrium | 89 | 1 | |
| 40 | Zr | zirconium | 90 | 0.5145(4) | g |
| 91 | 0.1122(5) | ||||
| 92 | 0.1715(3) | ||||
| 94 | 0.1738(4) | ||||
| 96 | 0.0280(2) | ||||
| 41 | Nb | niobium | 93 | 1 | |
| 42 | Mo | molybdenum | 92 | 0.146 49(106) | g |
| 94 | 0.091 87(33) | ||||
| 95 | 0.158 73(30) | ||||
| 96 | 0.166 73(8) | ||||
| 97 | 0.095 82(15) | ||||
| 98 | 0.242 92(80) | ||||
| 100 | 0.097 44(65) | ||||
| 43 | Tc | technetium | [97] | - | |
| 44 | Ru | ruthenium | 96 | 0.0554(14) | g |
| 98 | 0.0187(3) | ||||
| 99 | 0.1276(14) | ||||
| 100 | 0.1260(7) | ||||
| 101 | 0.1706(2) | ||||
| 102 | 0.3155(14) | ||||
| 104 | 0.1862(27) | ||||
| 45 | Rh | rhodium | 103 | 1 | |
| 46 | Pd | palladium | 102 | 0.0102(1) | g |
| 104 | 0.1114(8) | ||||
| 105 | 0.2233(8) | ||||
| 106 | 0.2733(3) | ||||
| 108 | 0.2646(9) | ||||
| 110 | 0.1172(9) | ||||
| 47 | Ag | silver | 107 | 0.518 39(8) | g |
| 109 | 0.481 61(8) | ||||
| 48 | Cd | cadmium | 106 | 0.012 45(22) | g |
| 108 | 0.008 88(11) | ||||
| 110 | 0.124 70(61) | ||||
| 111 | 0.127 95(12) | ||||
| 112 | 0.241 09(7) | ||||
| 113 | 0.122 27(7) | ||||
| 114 | 0.287 54(81) | ||||
| 116 | 0.075 12(54) | ||||
| 49 | In | indium | 113 | 0.042 81(52) | |
| 115 | 0.957 19(52) | ||||
| 50 | Sn | tin | 112 | 0.0097(1) | g |
| 114 | 0.0066(1) | ||||
| 115 | 0.0034(1) | ||||
| 116 | 0.1454(9) | ||||
| 117 | 0.0768(7) | ||||
| 118 | 0.2422(9) | ||||
| 119 | 0.0859(4) | ||||
| 120 | 0.3258(9) | ||||
| 122 | 0.0463(3) | ||||
| 124 | 0.0579(5) | ||||
| 51 | Sb | antimony | 121 | 0.5721(5) | g |
| 123 | 0.4279(5) | ||||
| 52 | Te | tellurium | 120 | 0.0009(1) | g |
| 122 | 0.0255(12) | ||||
| 123 | 0.0089(3) | ||||
| 124 | 0.0474(14) | ||||
| 125 | 0.0707(15) | ||||
| 126 | 0.1884(25) | ||||
| 128 | 0.3174(8) | ||||
| 130 | 0.3408(62) | ||||
| 53 | I | iodine | 127 | 1 | |
| 54 | Xe | xenon | 124 | 0.000 95(5) | |
| 126 | 0.000 89(3) | ||||
| 128 | 0.019 10(13) | ||||
| 129 | 0.264 01(138) | ||||
| 130 | 0.040 71(22) | ||||
| 131 | 0.212 32(51) | ||||
| 132 | 0.269 09(55) | ||||
| 134 | 0.104 36(35) | ||||
| 136 | 0.088 57(72) | ||||
| 55 | Cs | caesium | 133 | 1 | |
| 56 | Ba | barium | 130 | 0.0011(1) | |
| 132 | 0.0010(1) | ||||
| 134 | 0.0242(15) | ||||
| 135 | 0.0659(10) | ||||
| 136 | 0.0785(24) | ||||
| 137 | 0.1123(23) | ||||
| 138 | 0.7170(29) | ||||
| 57 | La | lanthanum | 138 | 0.000 8881(71) | g |
| 139 | 0.999 1119(71) | ||||
| 58 | Ce | cerium | 136 | 0.001 85(2) | g |
| 138 | 0.002 51(2) | ||||
| 140 | 0.884 50(51) | ||||
| 142 | 0.111 14(51) | ||||
| 59 | Pr | praseodymium | 141 | 1 | |
| 60 | Nd | neodymium | 142 | 0.271 52(40) | g |
| 143 | 0.121 74(26) | ||||
| 144 | 0.237 98(19) | ||||
| 145 | 0.082 93(12) | ||||
| 146 | 0.171 89(32) | ||||
| 148 | 0.057 56(21) | ||||
| 150 | 0.056 38(28) | ||||
| 61 | Pm | promethium | [145] | - | |
| 62 | Sm | samarium | 144 | 0.0308(4) | g |
| 147 | 0.1500(14) | ||||
| 148 | 0.1125(9) | ||||
| 149 | 0.1382(10) | ||||
| 150 | 0.0737(9) | ||||
| 152 | 0.2674(9) | ||||
| 154 | 0.2274(14) | ||||
| 63 | Eu | europium | 151 | 0.4781(6) | g |
| 153 | 0.5219(6) | ||||
| 64 | Gd | gadolinium | 152 | 0.0020(3) | g |
| 154 | 0.0218(2) | ||||
| 155 | 0.1480(9) | ||||
| 156 | 0.2047(3) | ||||
| 157 | 0.1565(4) | ||||
| 158 | 0.2484(8) | ||||
| 160 | 0.2186(3) | ||||
| 65 | Tb | terbium | 159 | 1 | |
| 66 | Dy | dysprosium | 156 | 0.000 56(3) | g |
| 158 | 0.000 95(3) | ||||
| 160 | 0.023 29(18) | ||||
| 161 | 0.188 89(42) | ||||
| 162 | 0.254 75(36) | ||||
| 163 | 0.248 96(42) | ||||
| 164 | 0.282 60(54) | ||||
| 67 | Ho | holmium | 165 | 1 | |
| 68 | Er | erbium | 162 | 0.001 39(5) | g |
| 164 | 0.016 01(3) | ||||
| 166 | 0.335 03(36) | ||||
| 167 | 0.228 69(9) | ||||
| 168 | 0.269 78(18) | ||||
| 170 | 0.149 10(36) | ||||
| 69 | Tm | thulium | 169 | 1 | |
| 70 | Yb | ytterbium | 168 | 0.001 26(1) | g |
| 170 | 0.030 23(2) | ||||
| 171 | 0.142 16(7) | ||||
| 172 | 0.217 54(10) | ||||
| 173 | 0.160 98(9) | ||||
| 174 | 0.318 96(26) | ||||
| 176 | 0.128 87(30) | ||||
| 71 | Lu | lutetium | 175 | 0.974 01(13) | g |
| 176 | 0.025 99(13) | ||||
| 72 | Hf | hafnium | 174 | 0.001 61(2) | g |
| 176 | 0.0524(14) | ||||
| 177 | 0.1858(9) | ||||
| 178 | 0.2728(6) | ||||
| 179 | 0.1363(3) | ||||
| 180 | 0.3512(16) | ||||
| 73 | Ta | tantalum | 180 | 0.000 1176(23) | |
| 181 | 0.999 8824(23) | ||||
| 74 | W | tungsten | 180 | 0.0012(1) | |
| 182 | 0.2650(16) | ||||
| 183 | 0.1431(4) | ||||
| 184 | 0.3064(2) | ||||
| 186 | 0.2843(19) | ||||
| 75 | Re | rhenium | 185 | 0.3740(5) | |
| 187 | 0.6260(5) | ||||
| 76 | Os | osmium | 184 | 0.0002(2) | g |
| 186 | 0.0159(64) | ||||
| 187 | 0.0196(17) | ||||
| 188 | 0.1324(27) | ||||
| 189 | 0.1615(23) | ||||
| 190 | 0.2626(20) | ||||
| 192 | 0.4078(32) | ||||
| 77 | Ir | iridium | 191 | 0.3723(9) | |
| 193 | 0.6277(9) | ||||
| 78 | Pt | platinum | 190 | 0.000 12(2) | |
| 192 | 0.007 82(24) | ||||
| 194 | 0.328 64(410) | ||||
| 195 | 0.337 75(240) | ||||
| 196 | 0.252 11(340) | ||||
| 198 | 0.073 56(130) | ||||
| 79 | Au | gold | 197 | 1 | |
| 80 | Hg | mercury | 196 | 0.0015(1) | |
| 198 | 0.1004(3) | ||||
| 199 | 0.1694(12) | ||||
| 200 | 0.2314(9) | ||||
| 201 | 0.1317(9) | ||||
| 202 | 0.2974(13) | ||||
| 204 | 0.0682(4) | ||||
| 81 | Tl | thallium | 203 | [0.2944, 0.2959] | |
| 205 | [0.7041, 0.7056] | ||||
| 82 | Pb | lead | 204 | 0.014(6) | |
| 206 | 0.241(30) | ||||
| 207 | 0.221(50) | ||||
| 208 | 0.524(70) | ||||
| 83 | Bi | bismuth | 209 | 1 | |
| 84 | Po | polonium | [209] | - | |
| 85 | At | astatine | [210] | - | |
| 86 | Rn | radon | [222] | - | |
| 87 | Fr | francium | [223] | - | |
| 88 | Ra | radium | [226] | - | |
| 89 | Ac | actinium | [227] | - | |
| 90 | Th | thorium | 230 | 0.0002(2) | |
| 232 | 0.9998(2) | ||||
| 91 | Pa | protactinium | 231 | 1 | |
| 92 | U | uranium | 234 | 0.000 054(5) | |
| 235 | 0.007 204(6) | ||||
| 238 | 0.992 742(10) | ||||
| Z | E | Element | A | Representative isotopic composition | Notes |
Periodic Table Of Elements Isotopes

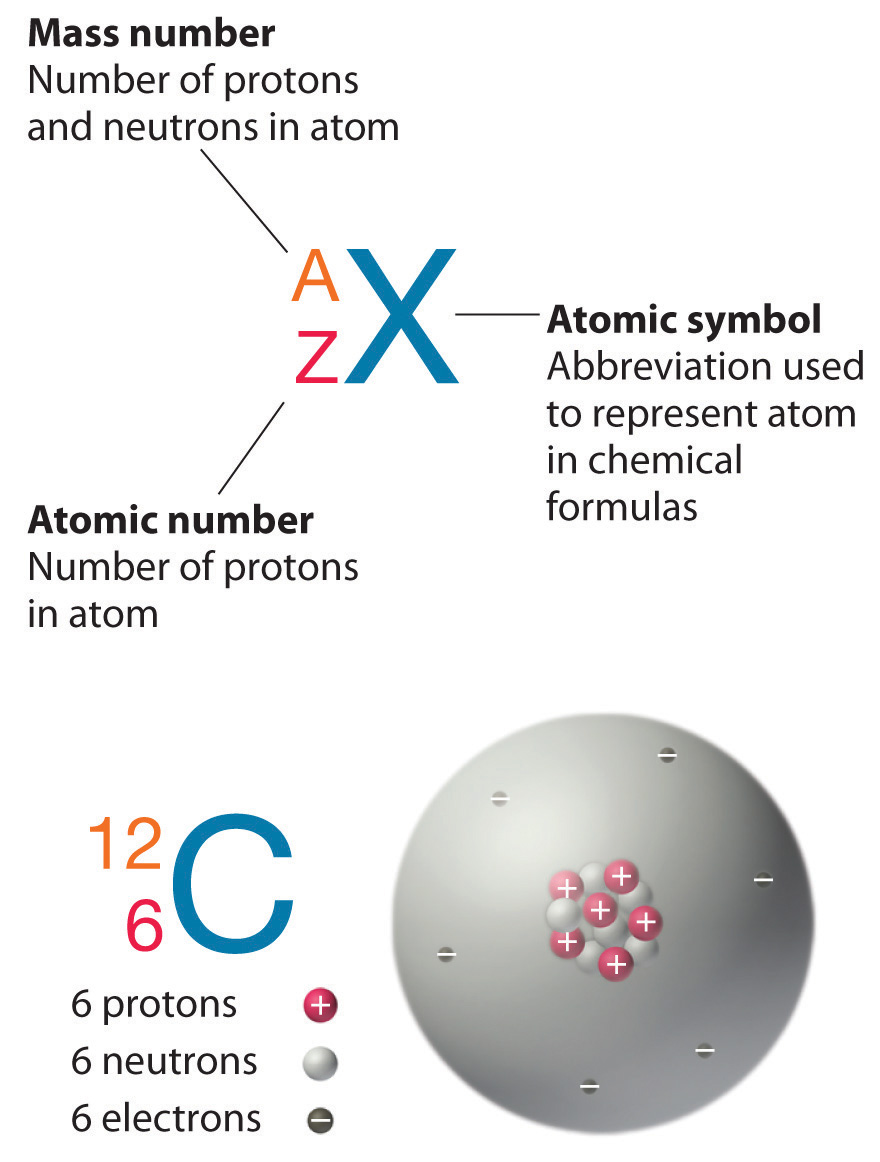
Isotope Element Chart
g Geological materials are known in which the element has an isotopic composition outside the limits for normal material. The difference between the atomic weight of the element in such materials and that given in the table may exceed the stated uncertainty.
m Modified isotopic compositions may be found in commercially available material because the material has been subjected to an undisclosed or inadvertent isotopic fractionation. Substantial deviations in atomic weight of the element from that given in the table can occur.
r Range in isotopic composition of normal terrestrial material prevents a more precise standard atomic weight being given; the tabulated atomic-weight value and uncertainty should be applicable to normal materials.
Isotopes Of Elements Diagram
Citation
Isotopes Of Elements Have Different Brainly
J. Meija et al
Isotopic compositions of the elements 2013Pure and Applied Chemistry88, 293-306 (2016)
This Table can be cited as follows:
CIAAW. Isotopic compositions of the elements 2019. Available online at www.ciaaw.org.
NOTE: Representative isotopic compositions of ytterbium, tantalum, iridium, argon, and hafnium were revised by CIAAW since the 'Isotopic compositions of the elements 2013' Report and these revisions are included in this table.
